URGENT – HAZARDOUS SITUATION – PLEASE REACT IMMEDIATELY
Dear CHPSO members,
Please be aware that the Institute for Safe Medication Practices has issued an URGENT ALERT regarding a hazardous packaging error. The following information is taken directly from their website:
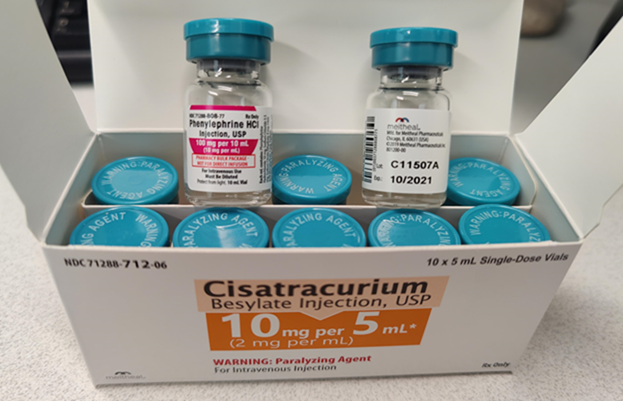
“ISMP is aware of an extremely hazardous packaging error involving certain cisatracurium products from Meitheal Pharmaceuticals. While the outer carton identifies the vials inside as cisatracurium, the vials contained in the carton are labeled phenylephrine injection. A photograph appears below. The vials actually contain cisatracurium and have caps appropriate for a paralyzing agent. However, this cap warning may not be noticed, since the vial is labeled phenylephrine.
Due to the nature of this situation and the potential for death if the these vials are used as phenylephrine injection in patients who are not intubated and ventilated, we urge facilities to immediately examine any and all cartons of cisatracurium from Meitheal Pharmaceuticals for this serious packaging error. The possibility that any of these vials were actually distributed should also be considered. ISMP has confirmed that both the FDA and the manufacturer are aware of this situation and that a recall is imminent.”
Questions or comments about this alert should be directed to Vivian Eusebio, CHPSO patient clinical advisor.